An international research project (Inter-Organ-PET) funded by the 2022 Joint ANR-FWF Projects Scheme between the Agence National de la Recherche (ANR) and the Austrian Science Fund (FWF) for “Identifying metabolic networks using inter-organ analysis of whole-body [18F]FDG-PET imaging data” (FWF I-6174B).
This project seeks to utilize whole-body molecular imaging by means of [18F]FDG-PET to assess inter-organ effects in breast cancer patients in comparison to normal, homeostatic pathways, and thus, help build prediction models for better breast cancer patient management.
Summary
The human body is a complex system acting on multiple interactions of numerous organs to ensure the organism’s proper functioning (homeostasis). Diseases may occur when such homeostatic balance supported by inter-organ communication is interrupted. Positron emission tomography (PET) is a widely available imaging method that can be used to examine the metabolic function of the human body non-invasively and quantitatively.
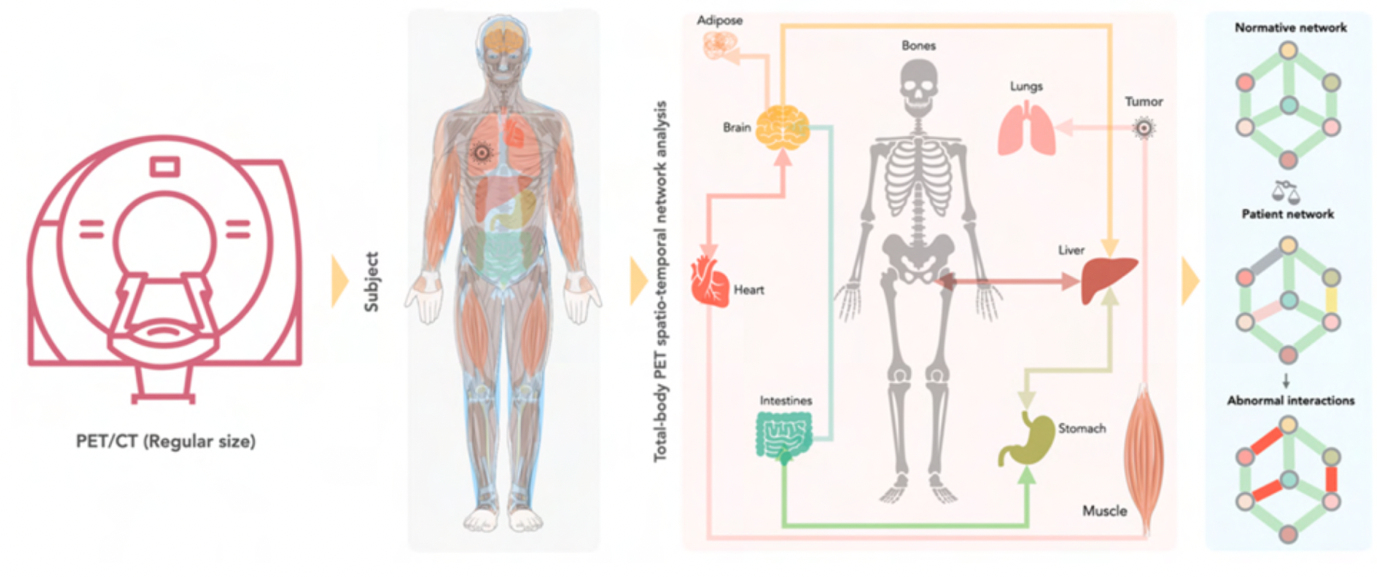
We can use PET imaging to examine the entire human body to see how organs “talk” with each other in healthy people and in patients with severe illnesses, such as breast cancer. Our hypothesis is that the exploration of the whole body may allow a better characterisation of the health status of these patients. We believe that metabolic pathways that are characteristic for cancer or tumour progression can be extracted from whole-body PET images.
In this very research project, we want to investigate how organs interact in patients with early and advanced breast cancer. We hope to generate cues about the prognosis and course of the disease in these patients. To do so, we first acquire PET image data of healthy subjects to reference inter-organ communications in the absence of severe disease. Then, we examine patients with breast cancer to understand if the organs interact differently in these patients.
Tracking the communication and interaction of multiple organs remains a challenging task, which requires the development of new methodologies and analytical tools. Therefore, scientists from different research fields (medicine, PET, artificial intelligence) have joined to advance our toolbox to explore the crosstalk between organs. If our new approach proves successful, we will be able to show the interaction between different organs in breast cancer patients for the first time. By doing so, we hope to assist clinicians in identifying the appropriate treatment from an increasing repertoire of therapeutics for an improved management of patients beat breast cancer.
Project Synopsis
Our understanding of diagnosis and effective treatment of complex diseases is limited. Recent studies point towards inter-organ axes communication and network effects that underpin numerous pathologies. Diagnostic molecular imaging (MI), including whole-body positron emission tomography (WB-PET), could support the non-invasive assessment of metabolic and signalling pathways on cellular and cross-/organ levels. WB-PET using [18F]-labelled glucose (FDG) as the tracer-of-choice offers unique opportunities to assess glycolytic pathways and to map interorgan signalling.
Through the simultaneous and quantitative characterisation of glycolic activity throughout the body, by means of whole-body [18F]-FDG PET imaging, we believe to assess inter-organ network effects non-invasively. Such endeavour entails the generation of an atlas of FDG-PET signals that are associated with non-pathological homeostatic metabolic networks in healthy subjects. Breast cancer is then considered as a perturbation of these networks, and we hypothesize that breast cancer is associated with inter-organ aberrations or altered network effects. It is understood that the appreciation of wider network effects requires an architecture of molecular and phenotypic networks for an in-depth understanding of disease expression and response to therapies. Nonetheless, we shall compare FDG-PET image-based aberrations as a function of disease stages (early, advanced) in breast cancer, and, thereby, hope to support predictive algorithms for better diagnostic management of breast cancer patients.
For updates on our research activities within this project, please follow us on LinkedIn.
Project Team and Centres
Coordinators
Thomas Beyer
P +43 (0)1 40400-39890