Research Area Representative
Francesco Moscato
P +43 (0)1 40400-39830
Research Groups
Cardiovascular Dynamics & Artificial Organs Group | Additive Manufacturing for Medical Research Group
More about this research area
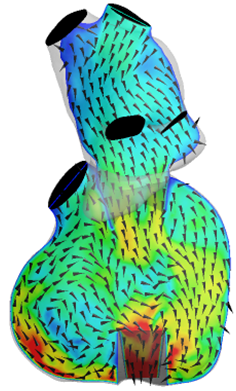
Local flow properties in the cardiovascular system are often correlated to diseases, adverse events and clinical outcomes. Therefore it is very important to understand overall and local fluid dynamics and interaction with the tissue and prostheses.
Diagnostic tools such e.g. clinical imaging systems are limited in their presentation of these important data. Therefore and also to understand variations of the clinical conditions, it is extremely important to perform in-silico and in-vitro simulations, which together with the clinical diagnostics allow testing of hypotheses of disease mechanisms, optimization of device design and a-priori investigations of therapeutic approaches.
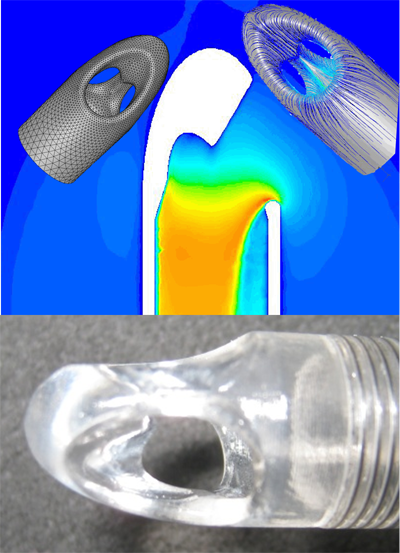
Over the years, our group has been developing multiple cardiac support devices and device components, including decades ago even a Total Artificial Heart for Clinical use, rotary pumps for different purposes, cannulae and peripheral components such as monitoring systems. Special focus is laid on biocompatibility and the human-system interface and its safety and usability.
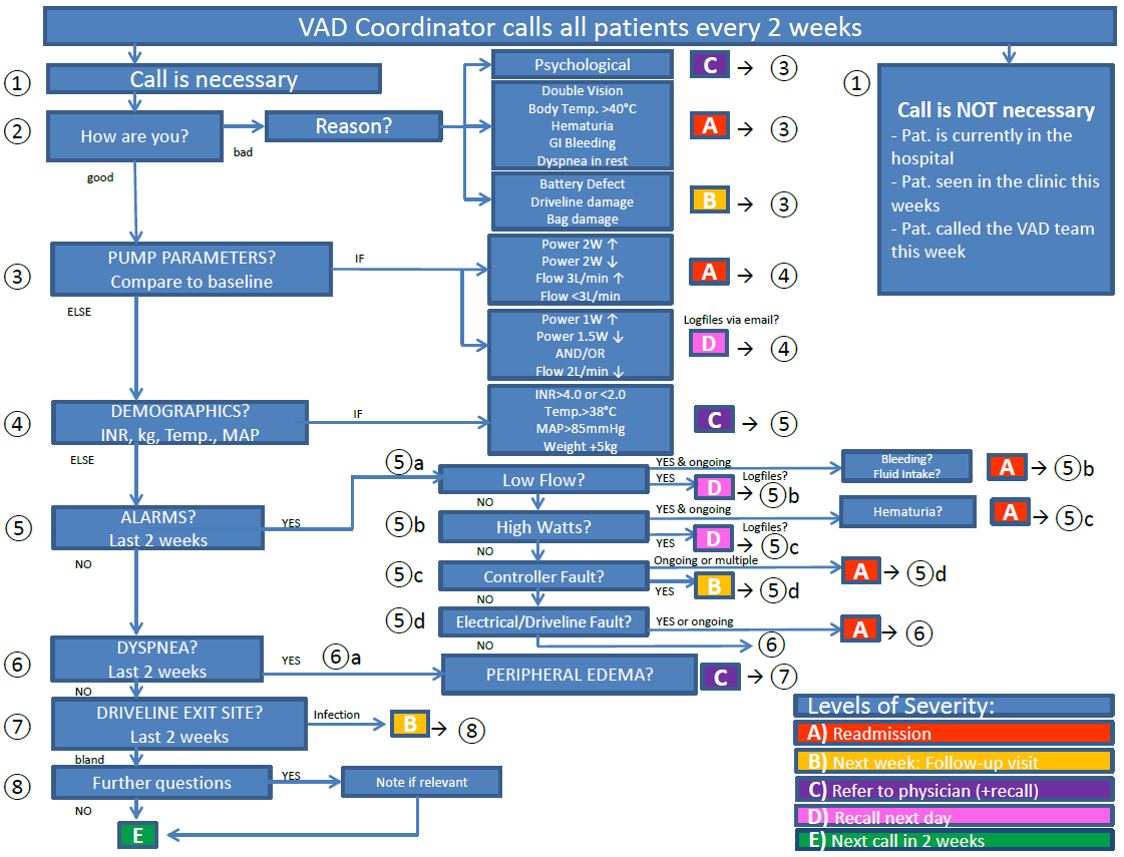
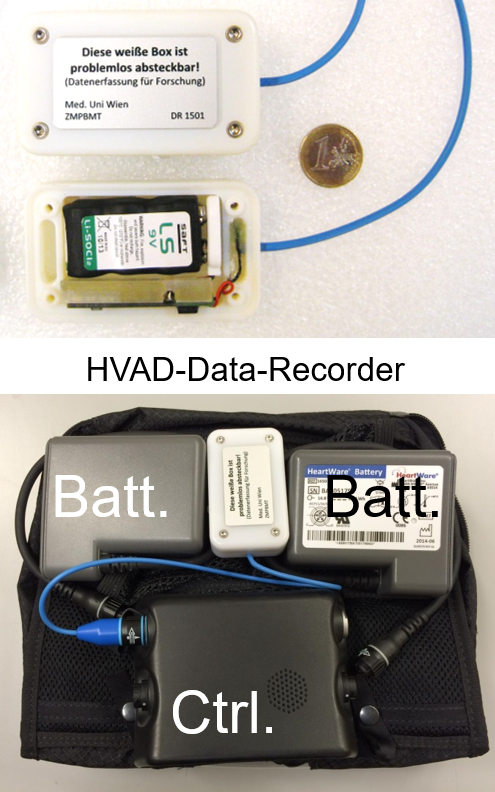
LVAD Outcomes Research Evidence Based Patient Management to Optimize Clinical Outcomes.
Ventricular Assist Devices (VADs) are an established therapeutic option for patients with chronic heart failure but adverse events (AEs), such as bleeding, infection, pump thrombosis (PT) and stroke have been associated with the use of these devices. The search for potential clinical risk factors and early predictors are of great interest to reduce AEs and hospital readmissions. Continuous monitoring and the adherence to guidelines and target ranges are essential to identify problems early and achieve optimal outcomes.
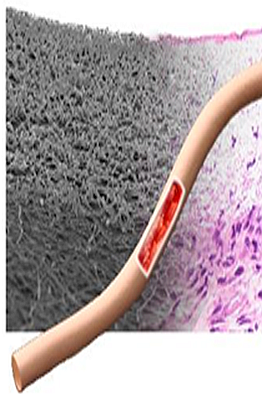
Electrospinning is a technique to manufacture small fibers (Ø 200nm – 2µm) from various materials.
Our group specializes in the spinning of biostable and biodegradable polymers from a dissolved state. The process has the potential to provide scaffolds with micro- to nanoscale topography and high porosity that is similar to the natural extracellular matrix and therefore useful in tissue engineering applications. The deposition of the fibers happens usually in a chaotic fashion. One of our aims is to increase the control the orientation of the deposited fibers.

After numerical and hydraulic simulation, our work must be validated in the biological setting.
Algorithms, procedures, diagnostic devices and implants have to be validated before the next steps to industry transfer and clinical application. To minimize unevitable animal experiments, our group has developed potent tools for in-vitro and ex-vivo-investigations, which are performed in the Center of Biomedical Research within close cooperation in the Boltzmann-Institute for Cardiovascular Research.
Static and dynamic characterization of biomaterials and tissues under physiological conditions.
In order to ensure that implants and medical devices are properly adapted to the organism, biomechanical tests are essential. Both for the characterization of biomaterials and their durability, as well as for information about biological tissues and their changes after surgery. These tests have to cover a wide range of operating points, from tiny structures that require special handling and measurement methods to large bones.
The cardiac activity is orchestrated by a complex interaction of hemodynamic, neural and hormonal control. Here we are concerned with understanding the influence of neural control on cardiac chronotropy, inotropy, and dromotrpy and with the development of neuroprostheses to restore and/or improve closed-loop cardiac neural control.